Research
The Oyakhire Lab studies and controls ion and electron transfer at solid-liquid interfaces in electrochemical systems. These interfacial charge-transfer processes determine the macroscopic performance of batteries, electrocatalytic cells, and related technologies. Consequently, designing next-generation energy storage and conversion devices depends on controlling charge transfer at interfaces.
We follow an iterative investigative cycle:
Synthesize hypothesis-informed materials such as thin films and small molecules using tunable methods.
Probe ion- and electron-transfer mechanisms to determine how these materials influence device properties such as efficiency.
Refine material design using the resulting mechanistic insights.
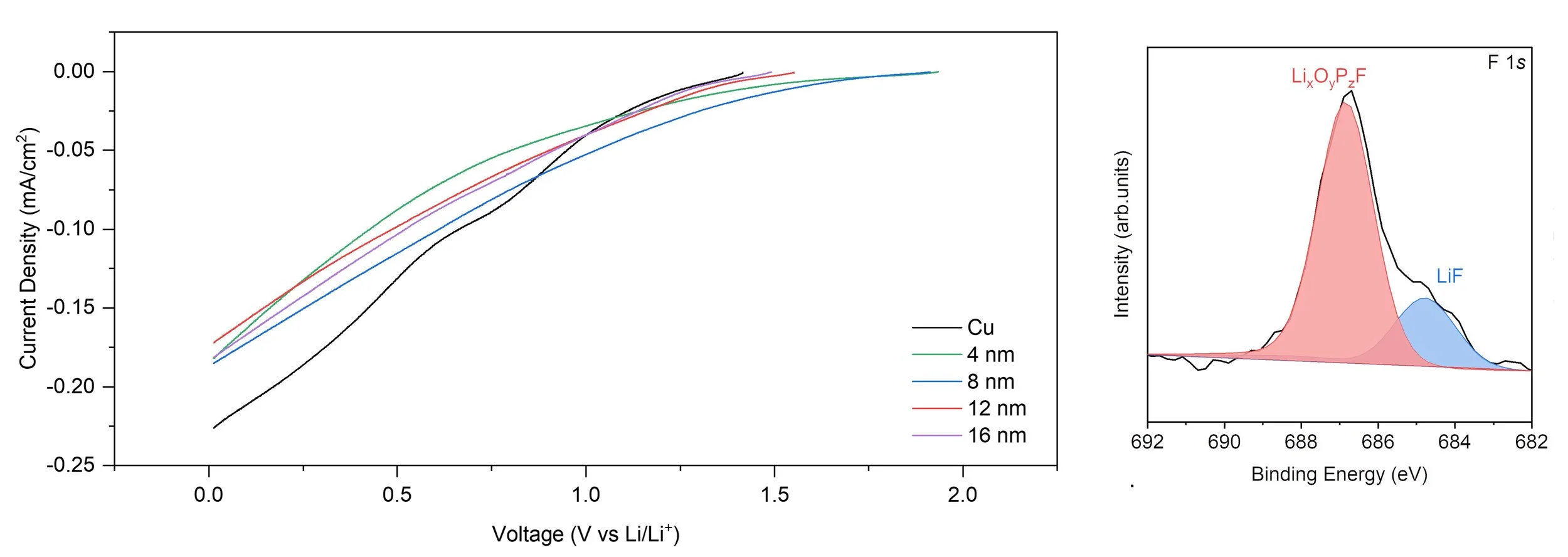
Electrodeposition in high-energy-density batteries
During charging, lithium- and sodium-metal batteries undergo electrically driven metal deposition and growth. Despite its central role in performance and safety, this process remains incompletely characterized. Using well-defined thin-film substrates, we elucidate key steps in metal growth within batteries, with the goal of enabling long-lasting, stable batteries.
Electrochemical resource recovery
Recycling minerals from waste is essential for environmental stewardship and equitable resource distribution. Conventional approaches such as chemical leaching, hydrometallurgy, and pyrometallurgy are limited in selectivity and efficiency. We exploit precise control over electron transfer and the broad tunability of electrochemical parameters to recover valuable resources from complex waste mixtures.
Tracking the evolution of electrically active interphases
The electric double layer (EDL), the solid electrolyte interphase (SEI), and the cathode–electrolyte interphase (CEI) mediate dynamic charge-transfer processes in batteries and electrocatalytic devices. Yet measurements of those inter(phases)faces have historically relied on static techniques. We develop operando spectroscopy and acoustic sensing methods to provide real-time insight into how these interfaces evolve, guiding the design of passivation layers that enhance battery cycle life.
Small-molecule discovery and design for electrochemical devices
In liquid cells, ion transport and electrode passivation are governed by the properties of small molecules. The vast design space of small molecules necessitates artificial-intelligence tools to identify optimal electrolytes. We develop Hypothesis-Informed, Physics-coupled Artificial Intelligence (HIP-AI) to design new molecules for batteries and resource-recovery cells. HIP-AI enables (i) discovery of new molecules through filtering and generative capabilities; (ii) interpretability via physics-coupled algorithms; and (iii) experimentally actionable outputs by linking experimental design parameters to HIP-AI features.